MEDIA
HELP
The eTMF is the cornerstone of the documentation of clinical trials. The content is created by CRO’s during the clinical trial, inspected by inspectors and finally handed over to the Sponsor. Despite the best effort of the International Conference on Harmonization (ICH) and the Drug Information Association (DIA) there is no TMF or eTMF standardization which could be used 1:1 by CROs, Sponsors and all stake holders. Even worse: This is the description of purpose of the DIA eTMF Reference Model by the DIA:
“The TMF Reference Model provides standardized taxonomy and metadata and outlines a reference definition of TMF content using standard nomenclature. The Model is not intended to be taken and used “of-the-shelf” but can be adapted to an electronic or paper TMF, and does not endorse, nor require, any specific technology for application. DIA members and industry members are under no obligation to adopt the TMF Reference Model.”
To summarize it. The TMF Reference Model
Based on the summary above no CRO and no Sponsor is bound to use a specific structure of an eTMF. There is no need that CROs and Sponsors use the standardized taxonomy, metadata, reference definition, standard nomenclature. Its s a good will to use the eTMF reference model.
CROs are often specialized on studies for specific therapy areas. Over the years the CROs have developed their own structure of an eTMF which might or might not follow the DIA eTMF reference model.
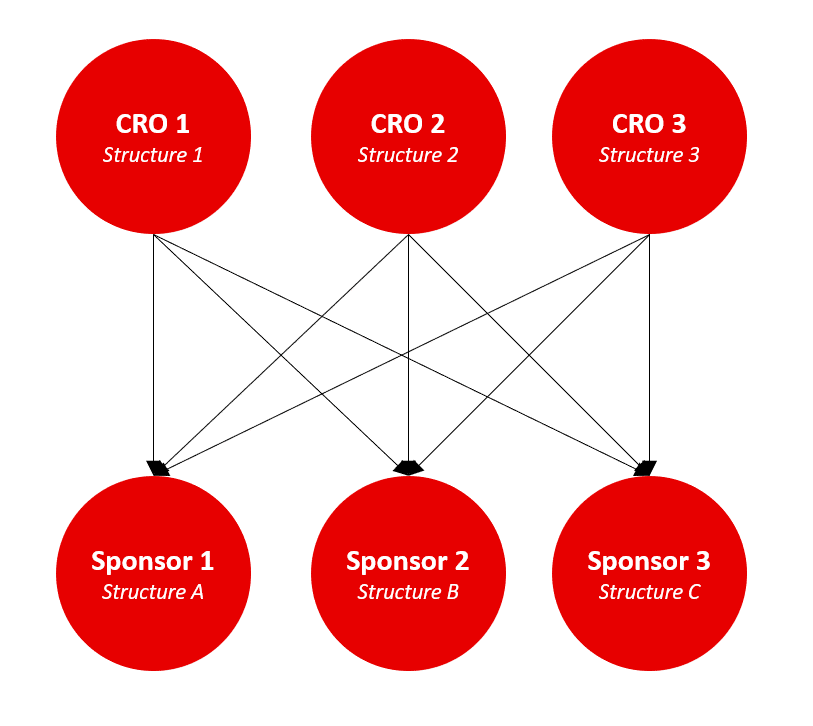
Different eTMF structures – both from CROs and Sponsors – result in labor intensive and costly adaptations for each clinical trial.
The CRO is using the own eTMF structure to document the clinical trial. If inspectors do not work with this CRO on a constant basis they have to analyze the structure of the eTMF to understand it. This is true for the Sponsors too: If the Sponsor is working with different CROs, for each clinical study the Sponsor gets a differently structured eTMF. Instead of having a structured form for all eTMFs, several structures must be understood and maintained. This is a very labor intensive and costly exercise. In addition, documents that are incorrectly sorted by the CRO, are difficult to find by the Sponsor. If incorrectly sorted documents are found during an inspection, this can lead to problems.
The use of an eTMF system does not help to improve the situation at all. The CROs implement the eTMF systems in a way that they reflect the structure of the paper TMFs. This means, that the eTMF in the eTMF system of the CRO just reflects the structure of the legacy paper TMF.
The dream of every Sponsor is to get the eTMF in a self-defined structure. Regardless of the structure in which the CRO created the eTMF. The easiest way to achieve this is to oblige the CRO to create the eTMF assembled during the clinical trial in the structure that the Sponsor needs. This leads to additional effort at the CRO. Consequently, the CRO is charging additional cost to the Sponsor to compensate the additional effort.
How could both parties (CROs and Sponsors) use the eTMF structure which suits best to their individual requirements?
The answer could be to connect the CRO and the Sponsor and put a restructuring layer in between these two parties. The restructuring layer transforms the structure of the eTMF of the CRO to the structure requested und used by the Sponsor. Both parties can work with the eTMF in a structure which suits them best without losing any information. Sponsors can easily use the eTMFs from earlier clinical trials to optimize planned studies.
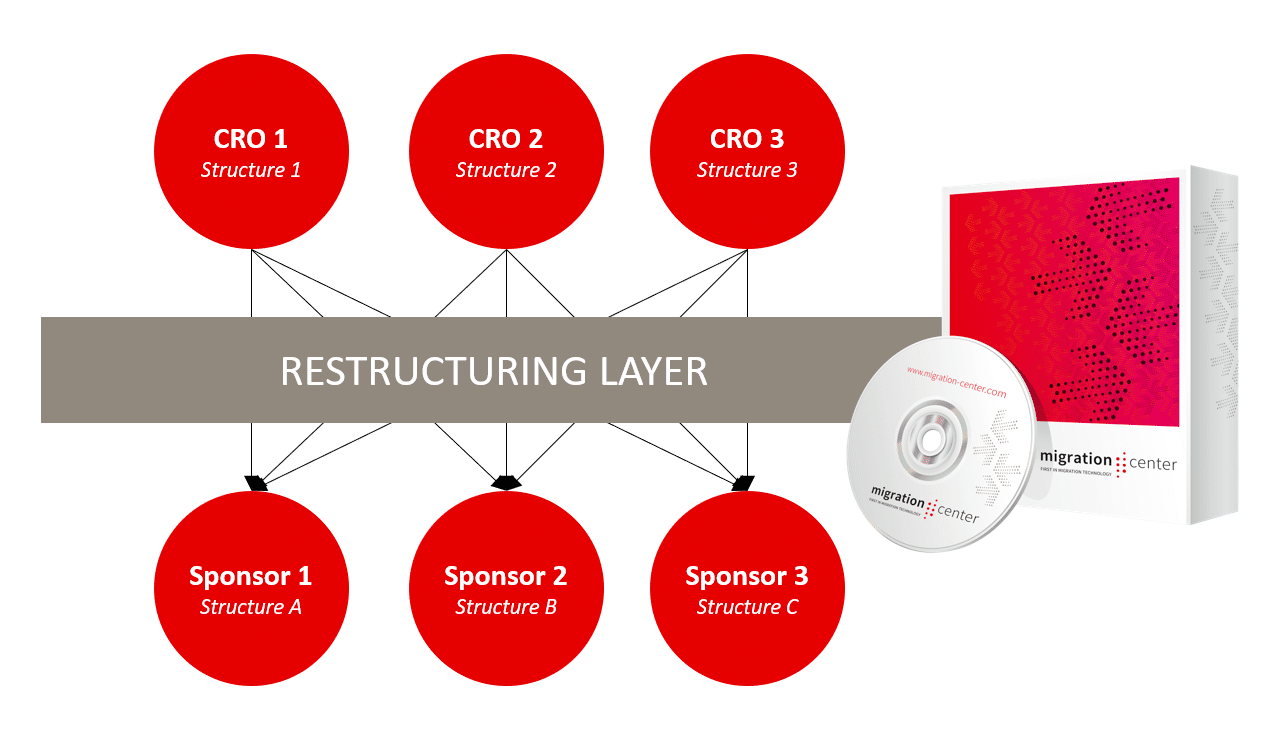
The restructuring layer of migration-center can adapt all the individual eTMF structures created by the CROs to the preferred eTMF structure of the Sponsors, thus saving substantial work and costs, reducing complexity and minimizing risk and failure.
The restructuring of the eTMFs can be easily done by using fme’s migration-center. Instead of migrating content from one content management system to another, the eTMF created by a CRO is migrated to the eTMF structure of the Sponsor. The complexity of handling the different structured eTMFs at the Sponsor is therefore reduced dramatically, by just using one eTMF structure.

 Information safekeeping – How to preserve information using the OAIS mode...
Information safekeeping – How to preserve information using the OAIS mode...